783
Views & Citations10
Likes & Shares
Cutaneous tuberculosis is an infection caused by M. tuberculosis complex, M. bovis and BCG vaccine, which depending on individual’s immunity, environmental factors and type of inoculum may present varied clinical and evolutionary aspect. Our study aims to review the current situation of cutaneous tuberculosis, to find out the incidence, clinical profile and histopathological features of cutaneous tuberculosis, atypical presentations if any, response to DOTS and highlighting the emergence of new and more specific methods of diagnosis.
Abbreviations: TB: Tuberculosis; BCG: Bacillus Calmette-Guérin; DOTS: Directly Observed Short course Therapy; ATT: Anti-Tuberculosis Therapy; CB-NAAT: Cartridge Based Nucleic Acid Amplification Test; MDR-TB: Multi-Drug Resistant Tuberculosis; DS & DR: Drug-Sensitive & Drug-Resistant; BPaMZ: Bedaquiline, Pretomanid, Moxifloxacin and Pyrazinamide
INTRODUCTION
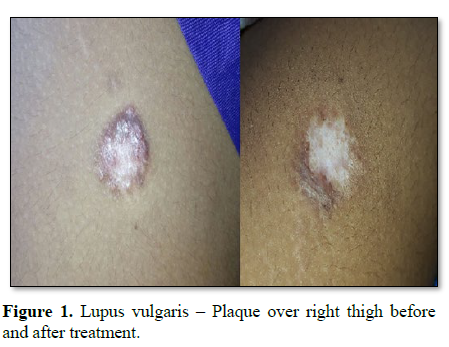
Cutaneous tuberculosis is an infection caused by M. tuberculosis complex, M. bovis and BCG vaccine, which depending on individual’s immunity, environmental factors and type of inoculum may present varied clinical and evolutionary aspects [1-3]. It has an incidence of around 5.9 cases per 1000 population. In India a tropical country, lupus vulgaris is the commonest type of secondary tuberculosis of skin in adults [4,5]. Renaissance of TB can occur due to the development of drug resistance, inappropriate treatment and poor health care.
Our study aims to review the current situation of cutaneous tuberculosis, to find out the incidence, clinical profile and histopathological features of cutaneous tuberculosis, atypical presentations if any, response to DOTS and highlighting the emergence of new and more specific methods of diagnosis.
One of our patients with atypical mycobacterial infections presented with chronic non healing ulcers over both upper limbs and trunk where in ulcers was mimicking cutaneous malignancies which were even negative in CB-NAAT testing. Patient also had chronic kidney disease with raised serum urea, creatinine on regular hemodialysis. He was started on therapeutic ATT trial with dose adjustment as per nephrologist opinion (reduction in 50% dose of ethambutol and pyrazinamide). Ulcers started to heal after initiating ATT in 3 weeks and later diagnosis was confirmed with culture. Since culture reporting takes a long time, there is a definitive role for therapeutic ATT trial when there is strong clinical suspicion. The duration of therapeutic trial should be 5-6 weeks, with exception of tuberculids and patients showing minimal clinical activity before treatment. Definitive diagnosis of MDR TB is difficult because of low sensitivity of molecular diagnostic methods and so a trial of ATT with second line drugs for at least 2 months before labeling a patient as non-responder.
In the early stages of evolution, TB and connective tissue disorder like SLE may mimic each other pretty much. In India which is set to be an abode of microbes, TB has to be ruled out prima facie by working out the case in depth to an extent of doing CB – NAAT (gene xpert) before starting on immunosuppressant. If the kind of vasculitic lesion is going to be Erythema Nodosum, ANA can be still false positive with increased CRP and ESR and could be misinterpreted as a case of SLE whereas the patient is really a case Pulmonary Koch with false positive ANA. In such a case scenario, if patient is started on immunosuppressant may land up in military tuberculosis and so every attempt has to be taken to distinguish between evolving SLE and TB.
The Gene xpert test is a molecular test for TB which diagnoses TB by detecting the presence of TB bacteria, as well as testing for resistance to the drug Rifampicin. In India, where this test is starting to be widely used, it is known as the CB-NAAT. The test is a molecular TB test which detects the DNA in TB bacteria. It uses a sputum sample and can give a result in less than 2 h. It can also detect the genetic mutations associated with resistance to the drug Rifampicin. Although culture gives a definitive diagnosis, to get the result usually takes weeks rather than the hours of the Gene xpert test. Bacterial culture still remains the gold standard test in diagnosis of non-tuberculous/atypical mycobacterial infections (Figure 3). CB-NAAT represents a major milestone for global TB diagnosis and care. Thus it helps millions of people who are at the highest risk of TB and drug resistant disease.
The recent approval of new tuberculosis (TB) drugs raises hope for new and more effective anti-tuberculosis treatment regimens. The Global Alliance for TB Drug Development (TB Alliance) is committed to ensuring that new anti-tuberculosis drugs fulfil the needs of patients, their families and the local health services that serve the communities. For the first time in four decades, new anti-tuberculosis drugs have reached regulatory approval, namely Bedaquiline and Delamanid. It is critical, however, that new drugs are not only approved by regulatory authorities, but that they are tested in trials where the results can be readily translated into meaningful changes in standards of care. SimpliciTB is a randomized clinical trial in which TB Alliance is testing a novel combination antimicrobial therapy called BPaMZ to determine an impactful treatment with the potential to transform TB therapy for both DS-TB and DR-TB.
CONCLUSION
Clinical diagnosis of early lesions of TB is often difficult, so correlation of clinical and histopathological features is a reliable tool for differentiating from its close mimics in most cases. Rarely Culture, CB-NAAT are needed when histopathological features are inconclusive but are useful tools which also give additional information like drug resistance. In doubtful cases, 5-6 weeks of therapeutic trial of ATT can be given. Cutaneous TB should be suspected in every case of chronic asymptomatic skin lesions. All Cutaneous TB patients must be evaluated completely for systemic involvement.
1. Azulay RD, Azulay DR (2011) Cutaneous tuberculosis. Rio de Janeiro, pp: 366-373.
2. Sampaio SAP, Rivitti EA (2008) Dermatologia: Tuberculosis and atypical mycobacteria. pp: 609-623.
3. Ministry of Health, Secretary of Health Surveillance (2008) National Guide for laboratorial surveillance of tuberculosis and other microbacteria. Brasília, DF.
4. Prasad PVS, Rao LL (1994) Lupus vulgaris with verrucosa. Indian J Dermatol Venereal Leprol 60: 347-348.
5. Rama Rao D (1993) Multiple lesions of lupus vulgaris with unusual morphology. Indian J Dermatol Venereal Leprol 59: 224-225.
6. Aruna C, Senthil Kumar AL, Sridevi K, Swapna K, Ramamurthy DVSB (2017) A clinico-epidemiological study of cutaneous tuberculosis in a tertiary care teaching hospital in Andhra Pradesh, India. Int J Res Dermatol 3: 88-93.
7. Ramam M, Tejasvi T, Manchanda Y, Sharma S, Mittal R (2007) What is the appropriate duration of a therapeutic trial in cutaneous tuberculosis? Further observations. Indian J Dermatol Venereol Leprol 73: 243-246.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)